
[ad_1]
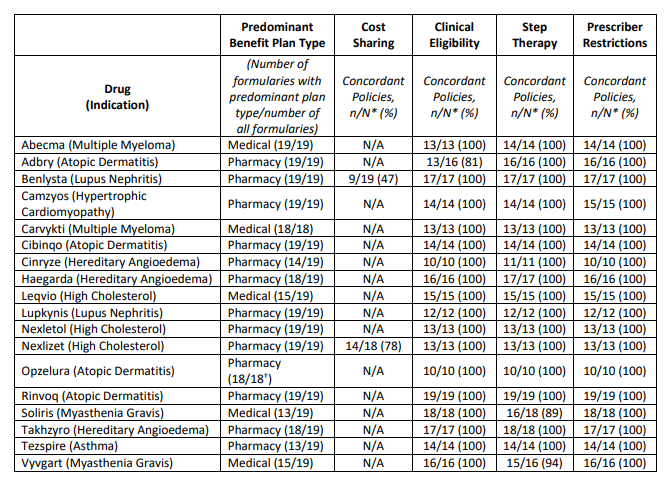
Final month, ICER launched their 2023 “Evaluation of Limitations to Truthful Entry” . The report concludes the next relating to 18 medication evaluated.
ICER defines “truthful entry” based mostly on the next standards:
Value sharing
- Value sharing based mostly on web worth. Affected person price sharing must be based mostly on the web worth to the plan sponsor, not the unnegotiated listing worth.
- No price for prime worth therapies. All medicines recognized by the Inner Income Service as high-value therapies ought to obtain pre-deductible protection inside excessive deductible well being plans.
- One low-cost choice out there in every class. At the least one drug in each class must be lined on the lowest related cost-sharing stage until all medication are priced greater than a longtime truthful worth threshold.
- Alright to have excessive price sharing if no medication are cost-effective. If all medication in a category are priced so that there’s not a single drug that represents a good worth as decided by worth evaluation, it’s affordable for payers to have all medication on the next costsharing stage.
- If all medication are priced at truthful worth, formulary placement is suitable. If all medication in a category are priced in order that they signify a good worth, it stays affordable for payers to make use of preferential formulary placement with tiered price sharing to assist obtain decrease total prices.
- Restricted cost-sharing if step-through required. As a part of financial step remedy, when sufferers attempt a decrease price choice with a decrease cost-sharing stage however don’t obtain an ample scientific response, price sharing for additional therapies must also be on the decrease cost-sharing stage so long as these additional therapies are priced pretty in keeping with clear standards.
Though ICER listing six standards, solely three (#3, #4, and #5) are formally assessed of their report.
Scientific eligibility
- Payers ought to supply options to prior authorization protocols similar to packages that give suggestions on prescribing patterns to clinicians or exempt them from prior authorization necessities (“gold carding”) in the event that they show excessive constancy to evidence-based prescribing.
- Payers ought to doc no less than as soon as yearly that scientific eligibility standards are based mostly on top quality, up-to date proof, with enter from clinicians with expertise in the identical or comparable scientific specialty.
- Scientific eligibility standards must be developed with express mechanisms that require payer workers to doc that they’ve: (i) thought-about limitations of proof on account of systemic under-representation of minority populations; and (ii) sought enter from scientific consultants on whether or not there are distinctive advantages and harms of therapy which will come up for organic, cultural, or social causes throughout completely different communities; and (iii) confirmed that scientific eligibility standards haven’t gone past affordable use of scientific trial inclusion/exclusion standards to interpret or slender the FDA label language in a method that disadvantages sufferers with underlying disabilities unrelated to the situation being handled
- For all medication: Scientific eligibility standards that complement the FDA label language could also be used to: (i) set requirements for prognosis; and/or • Outline indeterminate scientific phrases within the FDA label (e.g., “moderate-to-severe”) with express reference to scientific tips or different requirements; and/or (ii) triage sufferers by scientific acuity when the payer explicitly paperwork that triage is each affordable and crucial
- For medication with costs or worth will increase which were deemed affordable: Apart from the three functions outlined above, scientific eligibility standards mustn’t deviate from the FDA label language in a fashion that would chop protection.
- For medication with costs or worth will increase which were deemed affordable: Documentation that sufferers meet scientific eligibility standards ought to signify a lightweight administrative burden, together with acceptance of clinician attestation in lieu of extra formal medical file documentation until documentation is important to make sure affected person security.
- For medication with costs or worth will increase which were deemed unreasonable: Scientific eligibility standards might slender protection by making use of particular eligibility standards from the pivotal trials used to generate proof for FDA approval if carried out with affordable flexibility and supported by strong appeals procedures as described within the implementation standards.
Step Remedy and Switching
- As a way to justify financial step remedy insurance policies extending past FDA labeling as applicable, payers ought to explicitly affirm or current proof to doc the entire following: • Use of the first-step remedy reduces total well being care spending, not simply drug spending
- The primary-step remedy is clinically applicable for all or almost all sufferers and doesn’t pose a larger threat of any vital facet impact or hurt.
- Sufferers may have an affordable probability to fulfill their scientific objectives with first-step remedy.
- Failure of the first-step drug and the ensuing delay in starting the second-step agent is not going to result in long-term hurt for sufferers.
- Sufferers usually are not required to retry a first-line drug with which they’ve beforehand had adversarial negative effects or an insufficient response at an affordable dose and period.
- As a way to justify required switching insurance policies as applicable, payers ought to explicitly affirm or current proof to doc the entire following: (i) use of the required drug reduces total well being care spending. (ii) the required change remedy is predicated on the identical mechanism of motion or presents a comparable threat and facet impact profile to the index remedy. (iii) the required change remedy has the identical route of administration or the distinction in route of administration will create no vital unfavorable influence on sufferers on account of scientific or socio-economic elements. and (iv) sufferers usually are not required to modify to a drug that they’ve used earlier than at an affordable dose and period with insufficient response and/or vital negative effects, together with earlier use underneath a special payer
Supplier {qualifications}
- Restrictions of protection to specialty prescribers are affordable with a number of of the next justifications: Ii) correct prognosis and prescription require specialist coaching, with the chance that non-specialist clinicians would prescribe the treatment for sufferers who might undergo hurt or be unlikely to profit. (ii) willpower of the dangers and advantages of therapy for particular person sufferers requires specialist coaching on account of potential for severe negative effects of remedy. (iii) dosing, monitoring for negative effects, and total care coordination require specialist coaching to make sure secure and efficient use of the treatment.
- Requiring that non-specialist clinicians attest they’re caring for the affected person in session with a related specialist is an affordable choice when the situation is ceaselessly handled in major care settings however some parts of dosing, monitoring for negative effects, and/or total coordination of care would profit from specialist enter for a lot of sufferers
Truthful Entry Standards
- Value-sharing insurance policies must be offered clearly to shoppers previous to well being plan choice, permitting all people to grasp what price sharing they may face for remedies they’re at the moment taking or are contemplating.
- Any vital change to formulary or price sharing buildings mustn’t happen mid-cycle until plan sponsors embrace this as a qualifying occasion permitting plan enrollees to modify plans.
- On the level of care, clinicians and sufferers ought to have the ability to quickly decide the cost-sharing necessities for any therapy together with price sharing for different options.
- People contemplating well being plan enrollment must be offered with clear data permitting them to grasp whether or not they meet the insurers’ scientific standards for the remedies they’re at the moment taking. The insurance policies must also set out the rationale behind them and be readily comprehensible.
- Clinicians and sufferers ought to have the ability to quickly decide the scientific standards for any therapy and consider the scientific rationale supporting these standards. The referenced scientific data must be available to the prescribing/ordering supplier and the general public.
- People contemplating well being plan enrollment must be offered with clear data permitting them to grasp whether or not the remedies they at the moment take or envision taking might be topic to non-medical step remedy or switching insurance policies.
- Clinicians, pharmacists, and sufferers ought to have the ability to quickly decide the necessities associated to step remedy and switching insurance policies and have the ability to simply view a full justification from the insurer.
- People contemplating well being plan enrollment ought to have the ability to simply discover data associated to protection standards, together with prescriber {qualifications}, for medication that they or members of the family are at the moment taking.
- Clinicians and sufferers ought to have the ability to quickly decide whether or not there’s a restriction on prescribing for any therapy. Insurers ought to present prepared help to major care clinicians in search of reference to a related specialist for session as wanted
You possibly can learn the total report right here.
[ad_2]